Denture Cleansers Affect The Mechanical Behavior Of Heat Polymerized Acrylic Resins
STATEMENT OF THE PROBLEM: Disinfection of the dental prosthesis is the prime requirement to prevent cross infection control. The disinfectant regimen used to disinfectant prosthesis should not adversely affect the properties of these prosthesis. Therefore the compatibility between prosthesis and the disinfectant must be considered prior to disinfection.
OBJECTIVE: The aim of this study was to examine the surface hardness of denture base resins after immersed in four commercially available denture cleansers solution.
STUDY DESIGN: In vitro experimental study.
METHODOLOGY: A Total of one hundred and four specimens of measuring 13 mm—4 mm were fabricated. These 104 specimens were randomly divided into six groups. Each group consists of 18 specimens. These groups were: baseline (0 day dry), artificial saliva (control);Fittydent denture cleanser tablets;Fixodentdenture cleanser tablets, polident and corega. After 60 days of immersion in their respective experimental group, the specimens were tested for Vickers microhardness with Vickers microhardness tester.Statistical analysis was carried out by using SPSS 16 version.
RESULTS: There was statistically significant difference in the surface hardness among all groups (p < 0.001) after 60 daysof immersion.
CONCLUSION: Surface hardness of denture base resins decreased after immersion in denture cleansers KEY WORDS: Denture base, Denture cleanser, Vickers microhardness.
HOW TO CITE: Amin F, Akram S, Shaikh AA. Denture Cleansers Affect the Mechanical Behavior of Heat Polymerized Acrylic Resins. J Pak Dent Assoc 2015; 24(2):87-92.
INTRODUCTION
Acrylic resins were introduced in 1936 as denture base material1.Amongst their characteristics, are good thermal conductivity, low permeability to oral fluids, easy handling and colour stability1. One of the major disadvantage of these materials is that they can be deeply colonized by microorganisms.
Like cadida albicans, Streptococcus sanguis, Streptococcus salivarius, Streptococcus mutans, Fusobacterium nucleatum and Actinomyces viscosus2 These microorganisms transmitted from the contaminated devices between dental personnel and patients3. Most common opportunistic infection that is seen in denture wearers is denture-related stomatitis which is causedby the accumulation of denture plaque on prosthesis4. In the elderly patients there is a major risk of developing respiratory tract infection because of these microorganisms5.To prevent cross infection of microorganisms from contaminated prosthesis to dentists, dental assistant and patients, disinfection of theses prosthesis has been recommended as an vital procedure. For denture disinfection many procedures have been suggested. They are immersion in solutions6-15 and irradiation16-20. A study established an effective infection control protocol for denture disinfection by immersing the prosthesis in 3.78% solution of sodium perborate after scrubbing with 4% chlorhexidine10.In a preliminary study, the researchers immersed the specimens in water and then disinfection was carried out with microwave irradiation of 3 hard reline resins and they found that this regimen was effective against both pathogenic and nonpathogenic microorganisms17. Many chemical cleansers that contain sodium hypochlorite, acid solutions, enzymes, and alkaline peroxide, are available to remove the residual biofilm attached to denture surfaces15,21-23. Many studies demonstrated that denture cleansing solutions have antimicrobial properties22-27; however, none of these methods seem to effectively prevent recolonization on the denture surface and to remove the biofilm21-22. There is a wide variation in the literature regarding the use of denture cleansers as a study has shown that for reducing microorganisms the most effective denture cleanser is 0.5% sodium hypochlorite solution28. On the other hand in an another study researchers evaluate the maxillary dentures by usingalkaline peroxide tablets that there is significant decrease in Candida albicans colonyforming units as well as other29 microbial bioburden30. Surface hardness of the denture base polymers can be affected by dentifrice toothbrush/ abrasion,31 denture cleansers32, polymerization cycles,33 different systems used for polymerization of denture base34 and thermal cycling35. When acrylic resins were disinfected by chlorhexidine gluconate, sodium hypochlorite or sodium perborate, lower hardness values were observed36. According to Previous studies the hardness of the resins was not affected by immersing the conventional denture base polymer in 1% sodium hypochlorite, 4%
chlorhexidine solutions or in sodium perborate14,37. On the other hand the hardness can be altered by solutions of 2% glutaraldehyde , 4% chlorhexidine or 1% sodium hypochlorite38. Therefore the aim of this study is to assess the surface hardness of acrylic resins after immersed in different denture cleansers solution. The hypothesis of this in vitrostudy was that thesurface hardness of denture base resins was decreased after disinfection with denture cleansers.
METHODOLOGY
Table I presented the material used in this study. For the fabrication of samples Vertex¢ Rapid Simplified Holland was used that represent the conventional denture base acrylic resins. Fabrication and testing of specimens was conducted at Dr IshratulEbad Khan institute of Oral Health Sciences (Department of Science of Dental Materials and Department of Prosthodontics). Test specimens were fabricatedby the investment of material in stainless steel mold 13 x 4 mm. The American society for testing and material standard D 256-O6a was used for the dimension of specimens. The material was mixed accordingto the instructions by the manufacturer and after dough stage achieved, material was inserted into the molds. Specimens were inspected visually for any defects or porosities. Only those specimens were
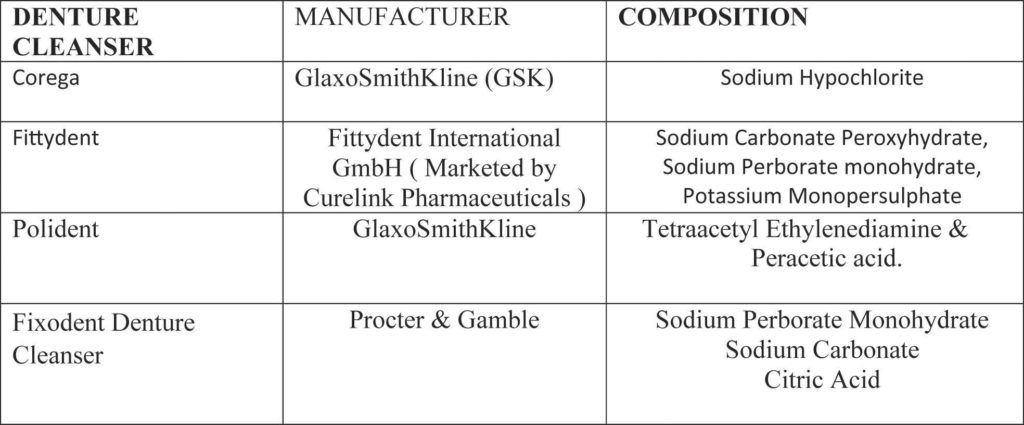 Table 1: Material used in the study
Table 1: Material used in the study
included in the studywhich were free of voids or porosity and having smooth surfaces. Excess material (flash) was removed immediately after polymerization and then polishing was done using progressively finer grades by silicon carbide paper (3M of Brazil; So Paulo, Brazil) for smooth, flat surface. Sample size was estimated by considering;Neppelenbroek KH et al17 work. A total of 108 specimens were fabricated. These specimens were divided randomly 06 groups (n=18). These groups were: at baseline (0 day dry specimens), artificial saliva (control) group. The experimental solutions of the study were: fittydent denture cleanser tablets, corega denture cleansers tablets, polidentdenture cleansers tablets and fixodent Denture Cleanser for 10 minutes according to manufacturer recommendations (Table 2). The samples were distributed
 Table 2: Denture cleansers used in the study
Table 2: Denture cleansers used in the study
through non probability convenience sampling. All specimens were placed in their respective containers and filled with distilled water except the specimens of baseline group. The specimens in the baseline group were measure at 0 day. The distilled water was discarded after 24 hours. The containers were then filled with their respective denture cleansers and artificial saliva. The specimens were washed and stored in distilled water. This disinfection regime was repeated twice a day for total of 60 days.
 Figure 1 : Micro Vickers Hardness Wolpert W group micro Vickers hardness tester digital autoturret.
Figure 1 : Micro Vickers Hardness Wolpert W group micro Vickers hardness tester digital autoturret.
Model number 402MVD
 Figure 2 : Specimen in Vickers hardness machine
Figure 2 : Specimen in Vickers hardness machine
 Figure 3: Surface morphology of heat cure acrylic taken from microscopic optic.
Figure 3: Surface morphology of heat cure acrylic taken from microscopic optic.
When no disinfection was carried out during storage specimens were placed in distilled water. The artificial saliva consisted of NaCl (0.40 g), KCl (0.4 g), NaOH (0.05 g), CaCl2·2H2O(0.22 g), NaH2PO4 (0.12 g), urea (1 g) in 1 dm3 of distilled water, adjusted to pH7. After 60 days, microhardness was evaluated using Vickers microhardness tester (Wolpert W group micro Vickers hardness tester digital auto-turret model number 402 MVD Figure 1,2 and 3). Data analysis was performed by using Statistical Package for Social Sciences (SPSS) version-16. The data was analyzed by using one way analysis of variance-one way (ANOVA) which was followed by Tuckey’s HSD (Honestly significant difference) was used at 0.05 significance level.
RESULTS
The mean values of the specimensat baseline, artificial saliva (control) and when immersed in experimental solutions were shown in Table 3. The corega denture
 Table 3: Mean and standard deviation of Vickers micro hardness values at baseline (0 day), artificial saliva (control) and after 60 days immersion in experimental groups.
Table 3: Mean and standard deviation of Vickers micro hardness values at baseline (0 day), artificial saliva (control) and after 60 days immersion in experimental groups.
cleanser tablets showed lowest value of hardness as compared to baseline and artificial saliva(control) specimens. One way ANOVA showed that significant difference was observed among all the groups (p < 0.001). It was further confirmed by post-Hoc Tukey test (Dunnett test) which stated that significant difference was found when baseline and artificial saliva (control) was compared with Fittydent, corega and poildent denture cleanser tablets (p < 0.001).
DISCUSSION
The effect of exposure of disinfection methods on the properties of denture base materials is of prime importance Vickers microhardness of denture base resins after immersed in denture cleansers were evaluated in this study. One sodium hypochlorite based, two sodium perborate based, and one tetraacetylethylenediamine based denture cleansers in effervescent tablet form which are commonly used on the local market were included in the study. The hypothesis that denture cleansers may decrease the micro hardness of denture base resins was accepted. The results showed that there was a significant decrease in Vickers micro hardness when specimens were immersed in denture cleansers solution when compared with the specimens who were not immersed in control group. The denture cleansers are chemical soak-type products. Sodium-perborate decomposed to form nascent oxygen, sodium metaborate and hydrogen peroxide and transforms to an alkaline peroxide solution41.This peroxide solution has chemical as well as mechanical cleansing mechanism by releasing oxygen39.
The decrease in Vickers hardness observed in this study might because of active oxygen released by hydrogen peroxide and oxygen liberating solution at a certain soaking temperature in the present study39 in perborate containing denture cleansers tablets that is Fixodent and fittydent denture cleanser tablets.A similar study conducted by Machado et al39 in which author used sodium perborate as an immersion media and found that hardness of denture base was decreased significantly after seven days of immersion as compared to the control group which was distilled water. In the present study, instead of distilled water, artificial saliva was used and found that hardness was not affected significantly after immersion into artificial saliva.
Other important factor might be due to the plasticizing effect of chemicals. lowering the hardness of the acrylic resin denture base resins as compared with artificial saliva might be due to the plasticizing effects of chemicals present in denture cleansers. These chemicals ingredients diffused in between the polymer chains causing relaxation of these chains and subsequently affected the hardness of denture base resins18,40,12,41. The duration of immersion and the type of denture cleanser play an important role in affecting the properties of denture base. Consistent with previous research,41 the hardness of heat cure resins decreased after immersion in denture cleansers. When acrylic resins are immersed in cleansers, residual monomers release42 and water absorption occur simultaneously. These processes are diffusion controlled and time-dependent43. It has been demonstrated that both water41 and residual monomer42,44 molecules act as plasticizers, thus affecting the strength of polymerized resins. As stated by Takahashi et al45 if the constituents that leach out exert a lesser plasticizing effect than ingredients in the denture cleansers, the strength of polymers will decrease.
To evaluate the effect of chemical immersion on denture bases,in vivo studies should be carried out. Evaluation of the efficacy of chemical disinfection through long term clinical trials should be carried out in the future.As the material is subjected to compressive, thermal, tensile and shear stresses unfavorable oral environment should be probed in future studies.one of the most important limitations of the study is due to the availability of limited technical resources available Vickers microhardnesswas evaluated.
CONCLUSION
Within the limitations of this in vitro study, it was concluded that:
- Surface hardness of denture base resins decreased after immersion in denture cleansers.
- The most significant decrease in the surface hardness of heat cure acrylic resins was with corega denture cleanser tablets when compared to the specimens at baseline (dry) and control group. This was followed by Fixodent denture cleanser tablets.
- The least change in hardness was observed with Fittydent denture cleanser tablets and poildent denture cleansers tablets respectively after immersing the specimens for 60 days.
REFERENCES
- Kurtulmus H, et al. Effects of saliva and nasal secretion on some physical properties of four different resin materials. Med Oral Patol Oral Cir Bucal, 2010;15:969975.
- Glass RT, Bullard JW, Hadley CS, Mix EW, Conrad RS. Partial spectrum of microorganisms found in dentures and possible disease implications. J Am Osteopath Assoc 2001;101:92-49.
- Jafari AA, Tafti AF, Falahzada H, Yavari MT. Evaluation of presence and levels of contamination in pumice powder and slurry used in clinical dental laboratories. Middle East J Sci Res 2006;1:50-53.
- Barbeau J, Sguin J, Goulet JP, de Koninck L, Avon SL, Lalonde B, et al. Reassessing the presence of Candida albicans in denture-related stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:51-59.
- Sumi Y, Miura H, Sunakawa M, Michiwaki Y,Sakagami N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology 2002;19:25-29.
- Abelson DC. Denture plaque and denture cleansers: review of the literature. Gerodontics 1985;1:202-206.
- Crawford CA, Lloyd CH, Newton JP, Yemm R.Denture bleaching: a laboratory simulation of patients’ cleaning procedures. J Dent 1986;14:258-261.
- Shen C, Javid NS, Colaizzi FA. The effect of glutaraldehyde base disinfectants on denture base resins. J Prosthet Dent 1989;61:583-9.
- Asad T, Wattkinson AC, Huggett R. The effects of various disinfectant solutions on the surface hardness of an acrylic resin denture base material. Int J Prosthodont 1993;6:9-12.
- Pavarina AC, Pizzolitto AC, Machado AL, Vergani CE, Giampaolo ET. An infection control protocol: effectiveness of immersion solutions to reduce the microbial growth on dental prostheses. J Oral Rehabil 2003;30:532-536
- Haywood J, Wood DJ, Gilchrist A, Basker RM, Watson CJ. A comparison of three hard chairside denture reline materials. Part II. Changes in colour and hardness following immersion in three commonly used denture cleansers. Eur J Prosthodont Restor Dent 2003;11:165.
- Rodrigues Garcia RC, Joane Augusto de S Jr, Rached RN, Del Bel Cury AA. Effect of denture cleansers on the surface roughness and hardness of a microwave-cured acrylic resin and dental alloys. J Prosthodont 2004;13:173178.
- Devlin H, Kaushik P. The effect of water absorption on acrylic surface properties. J Prosthodont 2005;14:233238.
- Azevedo A, Machado AL, Vergani CE, Giampaolo ET, Pavarina AC, Magnani R. Effect of disinfectants on the hardness and roughness of reline acrylic resins. J Prosthodont 2006;15:235-242.
- Paranhos HF, Silva-Lovato CH, Souza RF, Cruz PC, Freitas KM, Peracini A. Effects of mechanical and chemical methods on denture biofilm accumulation. J Oral Rehabil 2007;34:606-612.
- Dixon DL, Breeding LC, Faler TA. Microwave disinfection of denture base materials colonized with Candida albicans. J Prosthet Dent 1999;81:207-214.
- Neppelenbroek KH, Pavarina AC, Spolidorio DM, Vergani CE, Mima EG, Machado AL. Effectiveness of microwave sterilization on three hard chairside reline resins. Int J Prosthodont 2003;16:616-620.
- Campanha NH, Pavarina AC, Vergani CE, Machado AL. Effect of microwave sterilization and water storage on the Vickers hardness of acrylic resin denture teeth. J Prosthet Dent 2005;93:483-487.
- Sartori EA, Schmidt CB, Walber LF, Shinkai RS. Effect of microwave disinfection on denture base adaptation and resin surface roughness. Braz Dent J 2006;17:195-200.
- Sartori EA, Schmidt CB, Mota EG, Hirakata LM, Shinkai RS. Cumulative effect of disinfection procedures on the microhardness and tridimensional stability of a poly(methyl methacrylate) denture base resin. J Biomed Mater Res B ApplBiomater 2008;86B:360-364.
- De Souza RF: de Freitas Oliveira Paranhos H, Lovato da Silva CH, Abu-Naba’a L, Fedorowicz Z, Gurgan CA. Interventions for cleaning dentures in adults. Cochrane Database Syst Rev 2009, 7.
- Vieira AP, Senna PM, Silva WJ, Del Bel Cury AA: Long-term efficacy of denture cleansers in preventing Candida spp. biofilm recolonization on liner surface. Braz Oral Res 2010, 24:342-348.
- Dhamande MM, Pakhan AJ, Thombare RU, Ghodpage SL: Evaluation of efficacy of commercial denture cleansing agents to reduce the fungal biofilm activity from heat polymerized denture acrylic resin: An in vitro study. ContempClin Dent 2012;3:168-172.
- De Freitas Fernandes FS, Pereira-Cenci T, da Silva WJ, Filho AP, StraiotoFG,Del Bel Cury AA: Efficacy of denture cleansers on Candida spp. Biofilm formed on polyamide and polymethyl methacrylate resins. J Prosthet Dent 2011;105:51-58.
- Da Silva PM, Acosta EJ, Pinto Lde R, Graeff M, Spolidorio DM, Almeida RS, Porto VC: Microscopical analysis of Candida albicans biofilms on heat-polymerised acrylic resin after chlorhexidine gluconate and sodium hypochlorite treatments. Mycoses 2011, 54:e712-717.
- De Andrade IM, Cruz PC, da Silva CH, de Souza RF, ParanhosHde F, Candido RC, Marin JM, de SouzaGugelmin MC: Effervescent tablets and ultrasonic devices against Candida and mutans streptococci in denture biofilm. Gerodontology 2011, 28:264-270.
- Sousa FA, Paradella TC, Koga-Ito CY, Jorge AO: Effect of sodium bicarbonate on Candida albicans adherence to thermally activated acrylic resin. Braz Oral Res 2009, 23:381-385.
- Sousa-Porta, S. R., Lucena-Ferreira, S. C., Silva, W. J. & Del Bel Cury, A. A. Evaluation of Sodium Hypochlorite as a Denture Cleanser: A Clinical Study,” Gerodontology, 2013.
- Uludamar, A., Ozkan, Y. K., Kadir, T. & Ceyhan, I. “In Vivo Efficacy of Alkaline Peroxide Tablets and Mouthwashes on Candida Albicans in Patients with Denture Stomatitis,” J Appl Oral Sci, 2010;18:291-296.
- Rossato, M. B., Unfer, B., May, L. G. & Braun, K. O. “Analysis of the Effectiveness of Different Hygiene Procedures Used in Dental Prostheses,” Oral Health Preventive Dent, 2011;9: 221-227
- Richmond, R., Macfarlane, T. V. &Mccord, J. F. “An Evaluation of the Surface Changes in PMMA Biomaterial Formulations as a Result of Toothbrush/Dentifrice Abrasion,” Dent Mat,2004;20:124-132
- Durkan, R., Ayaz, E. A., Bagis, B., Gurbuz, A., Ozturk, N. &Korkmaz, F. M. “Comparative Effects of Denture Cleansers on Physical Properties of Polyamide and Polymethyl Methacrylate Base Polymers,” Dent Mat J, 2013;32; 367-375.
- Lira, A. F., Consani, R. L. X., Mesquita, M. F., Nobilo, M. A. A. &Henriques, G. E. P. “Effect of Toothbrushing, Chemical Disinfection and Thermocycling Procedures on the Surface Microroughness of Denture Base Acrylic Resins,” Gerodontology 2012:29 E891-E897.
- Ali, I. L., Yunus, N. & Abu-Assan, M. I. “Hardness, Flexural Strength, and Flexural Modulus Comparisons of Three Differently Cured Denture Base Systems,” J Prostho, 2008;17:(7) 545-549.
- Goiato, M. C., Dos Santos, D. M., Baptista, G. T., Moreno, A., Andreotti, A. M. &Dekon, S. F”Effect of Thermal Cycling and Disinfection on Microhardness of Acrylic Resin Denture Base,”J Med Eng Technol. 2013 Apr;37:203-207.
- Neppelenbroek, K. H., Pavarina, A. C., Vergani, C. E. &Giampaolo, E. T. “Hardness of Heat-Polymerized Acrylic Resins after Disinfection and Long-Term Water Immersion,” J Prosth Dent,2005;93:171-176.
- Machado, A. L., Breeding, L. C., Vergani, C. E. Perez, L. E. C. “Hardness and Surface Roughness of Reline and Denture Base Acrylic Resins after Repeated Disinfection Procedures,” J Prosth Dent, 2009;102:115-122.
- Carvalho, C. F., Vanderlei, A. D., Marocho, S. M., Pereira, S. M., Nogueira, L. &Paes- Junior, T. J. “Effect of Disinfectant Solutions on a Denture Base Acrylic Resin,” Acta Odontol Latinoam, 2012;25:(3) 255-260.
- Yatabe M, Seki H, Shirasu N, Sone M. Effect of the reducing agent on the oxygen inhibited layer of the crosslinked reline material. J Oral Rehabil 2001; 28: 180-185.
- Azevido A, Machado AL, Pavarina AC, Vergani CE, Giampaolo ET. Hardness of denture base and hard chair side reline acrylic resins. J Appl Oral Sci.2005;13:291295.
- Pavarina AC, Vergani CE, Giampaolo ET, Machado AL, Teraoka MT. The effect of disinfectant solutions on the hardness of acrylic resin denture teeth. J Oral Rehabil.2003; 30:749-752
- Lee SY, Lai YL, Hsu TS. Influence of polymerization conditionson monomer elution and microhardness of autopolymerizedpolymethyl methacrylate resin. Eur J Oral Sci. 2002;110:179-183.
- Vallittu PK, Miettinen V, Alakuijala P. Residual monomer contentand its release into water from denture base materials. Dent Mater.1995;11:338-342.
- Braun KO, Mello JA, Rached RN, Del Bel Cury AA. Surface textureand some properties of acrylic resins submitted to chemical polishing.J Oral Rehabil. 2003;30:91-98.
- Takahashi Y, Chai J, Kawaguchi M. Equilibrium strengths of denture polymers subjected long-term water immersion. Int J Prosthodont 1999;12:348-352.

